The Compass
Sanjula Jain, Ph.D. | August 13, 2023The Cell and Gene Patient Journey Is Determined by Access to Treatment Sites
Key Takeaways
-
As the growing disease burden in the U.S. intensifies patient morbidity and acuity, demand for CGT is likely to accelerate. However, supply from both a treatment and workforce perspective is not prepared to meet current, much less future, demand.
-
Nationally, the sites of service for CGT administration are more concentrated in the Northeast and Midwest.
-
Many sites of care can only support the administration of a subset of CGTs. The range of therapies that a care site can provide depends on its resources and equipment for handling more intricate treatments like CAR-T cell therapies, its clinical trial status, as well as the medical specialties (e.g., cancer care) and availability of specialized staff at the site of care.
Certain areas of care delivery could potentially be transformed by the further development of cell and gene therapies (CGT), particularly Chimeric Antigen Receptor T-cell (CAR-T) therapy. CGT has the potential to revolutionize the treatment of multiple diseases—particularly blood and other cancers, immunodeficiencies, neurologic disorders and rare diseases. However, several barriers such as cost, time commitment, limited availability and drug shortages hinder widespread adoption and accessibility of CGT. As the growing disease burden in the U.S. intensifies patient morbidity and acuity, demand for CGT is likely to accelerate. However, supply from both a treatment and workforce perspective is not prepared to meet current, much less future, demand.
Background
Cell therapy involves the introduction of genetic material into cells to treat or prevent diseases by modifying the production of specific proteins within the cell. Gene therapy involves transplanting viable cells, derived either from the patient or a donor, into patients to alleviate or cure diseases. These cells are selected based on their potential to differentiate into various cell types.1 CAR-T cell therapy harnesses the body's immune cells to combat cancer by modifying immune cells to express specific receptors, which can recognize and attack cancer cells more effectively. CAR-T therapy is FDA-approved for treating leukemia, lymphoma and multiple myeloma and holds substantial promise to transform cancer care.2
Currently, there are 32 FDA-approved CGTs—six of which are CAR-T therapies. In 2019, the Food & Drug Administration predicted that it would approve between 10 and 20 new CGTs per year by 2025.3 Last year, three new CGTs were approved, and as many as 13 new CGTs could be approved for use in the US and/or Europe by the end of 2023.4
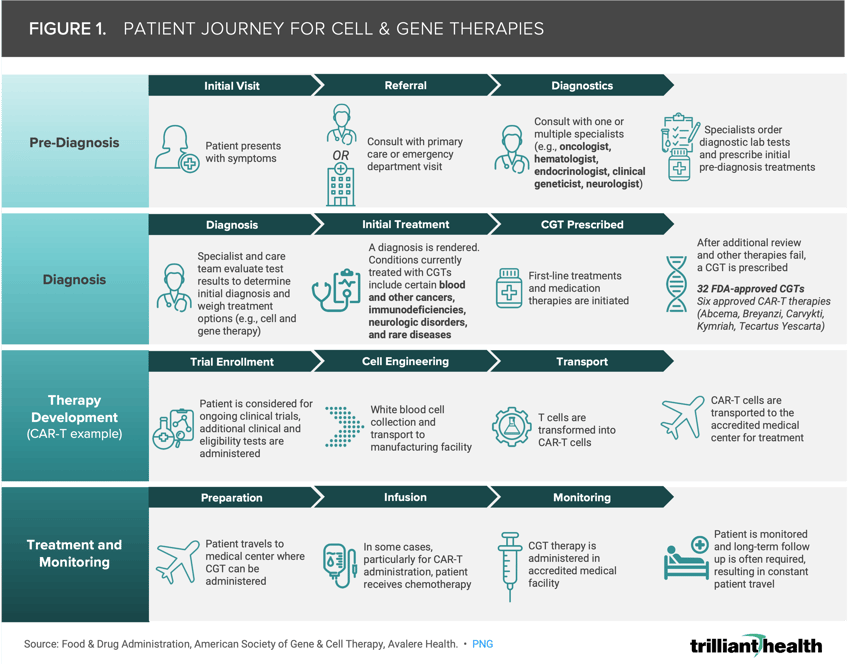
Figure 1 illustrates an example journey of a patient undergoing cell and gene therapy specifically resulting in CAR-T cell therapy. Patient journeys encompass a range of activities across pre-diagnosis, diagnosis, treatment planning, therapy administration and post-treatment monitoring. Although the target patient populations for CGTs are small, rare diseases affect up to 25M Americans.5
Numerous barriers hinder widescale adoption of these promising therapies, particularly drug and staff supply, demographic disparities, insurance coverage and affordability. Manufacturing capacity constrains the availability of therapies, causing delays and supply shortages.6 Additionally, staffing limitations—as evidenced by ongoing shortages in the healthcare workforce—challenge the delivery of these therapies, which require specialized training for administration.7,8 CGTs are some of the most expensive therapies on the market. Zolgensma, which launched in 2019 and treats spinal muscular atrophy, was the most expensive therapy to enter the U.S. market at the time with an initial list price of $2.1M.9
Socioeconomic factors, including affordability, transportation options and caregiver support, limit access for many patients. Traditionally, CGT/CAR-T is administered in an inpatient setting, typically at large academic medical centers, which often prevents otherwise eligible patients in rural areas from accessing these therapies.
State Medicaid programs—which are required to cover all FDA-approved drugs—have tried innovative approaches to manage CGT costs, including utilization management and innovative contracting, including managed and bundled payment care carve-outs.10 In contrast to typical adoption of innovative procedures and therapies, Medicare coverage of CGTs has lagged behind other coverage sources, although in February 2023 CMS suggested a bundled payment approach for Medicare to replace fee-for-service billing during CGT episodes of care. While commercial plans are not required to cover CGT, commercial plans commonly obtain primary and secondary reinsurance in order to control the high costs associated with CGT.11
Compounding these barriers, our research has found that patient acuity is intensifying. Data suggests the national quarterly share of new patients at oncology providers is stabilizing above pre-pandemic levels, signaling growth in prevalence of new post-pandemic cancer diagnoses.12 As a result, we were interested in better understanding the current CGT landscape. Given much of the existing literature is focused on individual therapies with less known about the location of service, we were interested in examining access patterns and the distribution of therapy services to inform the development of future strategies.
Analytic Approach
Using all-payer medical and pharmacy claims data and our national Provider Directory, we identified eight approved CGTs and their corresponding service sites since 2017. To analyze the geographic distribution of CGT access, we then mapped these sites of service nationally.
Findings
Nationally, the sites of service for CGT administration are limited (Figure 2), and 18 states are not represented among the top sites of service that administer these eight CGTs. Sites are more concentrated in the Northeast and Midwest, while patients in the West must travel significantly farther, on average, given relatively few treatment centers. Moreover, many sites of care can only support the administration of a subset of CGTs. The range of therapies that a care site can provide depends on its resources and equipment for handling more intricate treatments like CAR-T cell therapies, its clinical trial status, as well as the medical specialties (e.g., cancer care) and availability of specialized staff at the site of care.
It is well established that the COVID-19 pandemic disrupted diagnostic pathways—particularly cancer screening—for various diseases, including those targeted by CGT.13 Further research is needed to fully understand the extent of these disruptions and their long-term effects, some of which could suggest greater need for these therapies in the years ahead.
To overcome the challenges and enhance patient access to CGT, several interim solutions could be considered by different stakeholders. Implementing comprehensive patient support programs could address various barriers, including travel costs, compliance challenges and care coordination. These programs can offer crucial assistance during diagnostic monitoring and long-term follow-up.
Investments in training are also needed to equip healthcare staff with the skills needed to administer CGT to enable safer and more widespread therapy delivery. Health system and government partnerships could be formed to create incentives and policy frameworks that promote the equitable distribution of CGT. Such partnerships can help address disparities in access and affordability.
While CGTs hold the potential to not only treat but even cure certain cancers and rare diseases, the current supply—both in terms of treatment and providers—cannot meet the current demand, which is certain to grow as America ages and new therapies are approved. While CGTs may not be common standard of care, the more that we can quantify the impact these therapies on the total cost of care and downstream health outcomes of managing patients with rare disease, the more successful stakeholders will be in harnessing the value of CGTs.
Thanks to Sarah Millender, Austin Miller and Katie Patton for their research support.
- Social Determinants of Health
- Specialty Care
- Life Sciences
- Disease Burden
You are currently viewing a free preview of our premium studies. To receive new studies weekly, upgrade to Compass+ Professional.
Sign UpSee more with Compass+
You are currently viewing the free version of this study. To access the full study, subscribe to Compass+ Professional for $199 per year.
Sign Up for Compass+